Throughout this escape room, small LEDs were used, particularly in the lip stick torches. As with any light sources, the basic origin of the photons is an excited electron emits some of its energy in the form of light. This is also indicated by the name as LED is short for light emitting diode. Specifically for LEDs, an n-p junction is used. The n simply stands for negative, while the p stands for positive. The following schematic shows how this works.
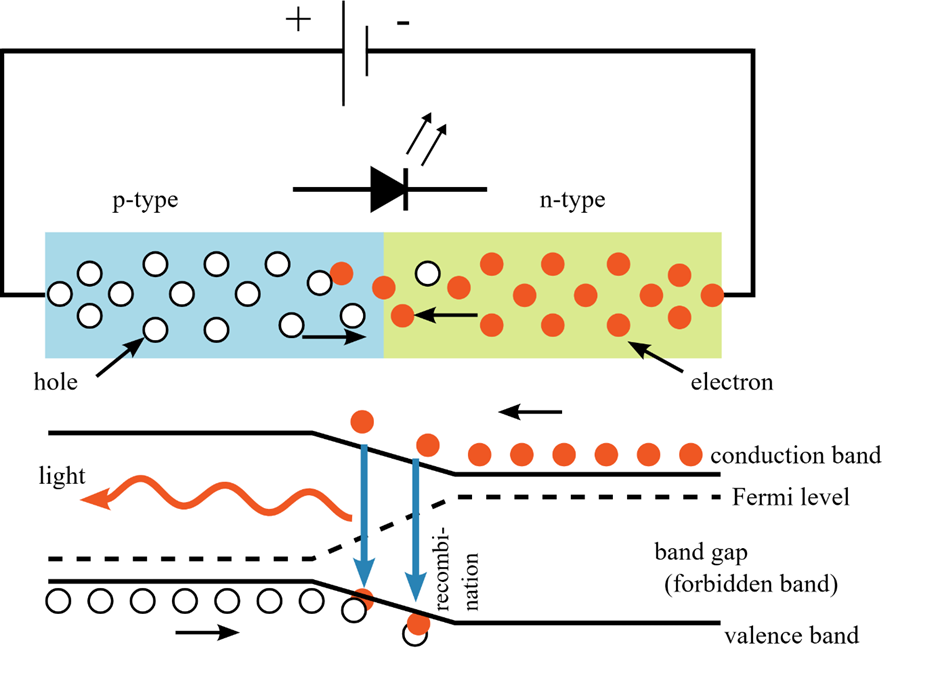
(source: Wikipedia By S-kei – Own work based on: PnJunction-LED-E.PNG, CC BY-SA 2.5, https://commons.wikimedia.org/w/index.php?curid=14985902)
On one side of the junction, the n-type, the electrons are supplied by a power source, while on the p-side holes are located. These are quantum mechanical objects that are basically the absence of electrons. The electrons want to fill these holes. However, the holes and the electrons have different energy levels, with the electrons initially in the conduction band, while the holes are in the valence band. This gap in energy needs to be overcome. To visualize this, one can think of the electrons like balls of yarn. The bigger the ball, the higher the energy. If you have holes that are to small, these balls do not fit through, unless some of the yarn is removed. This means that the electrons need to lose energy to “fill” the holes. So, the additional energy of electrons need to be dispersed somewhere. Partially, they are dispersed into heat, but more importantly and more substantially, it is dispersed into light. Moreover, the energy of light is proportional to its wavelength, so by varying the size of the gap (the energy difference), the wavelength is changed. As mentioned previously, the wavelength of light basically corresponds to the colour of light. Nevertheless, it isn’t equally easy to arrive at different gaps, so for a long time, there were no blue LEDs. The exact reasons for that are not important to understand the basic principle, but for those interested, a comprehensive history of blue LEDs is explained well in the following video: Veritasium: Blue LEDs. It also features another explanation of how LEDs work generally.
